
Neon Bohr model Science ShowMe
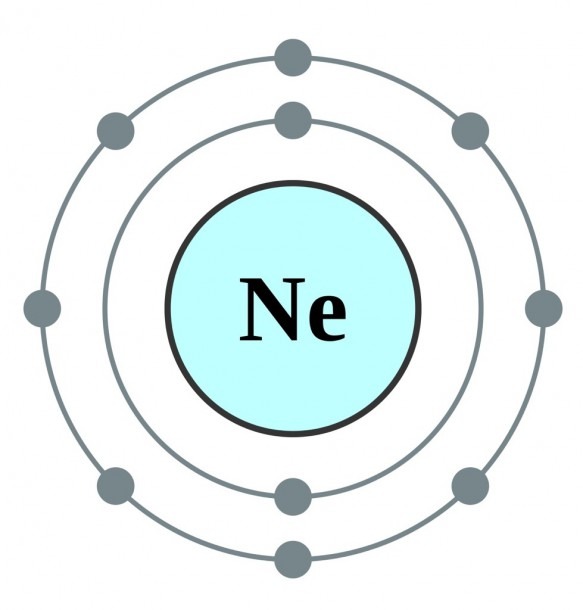
Neon has 2 electrons in its first shell and 8 in its secondCheck me out: http://www.chemistnate.com

Atomic Structure (Bohr Model) for the Neon (Ne) Atom YouTube
The drawbacks of the Rutherford model of the atom, including its apparent instability, would soon be addressed by his student, Dutch physicist Neils Bohr (1885 - 1962). To unlock this lesson you.

neon atomic structure neon atom diagram QFB66
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
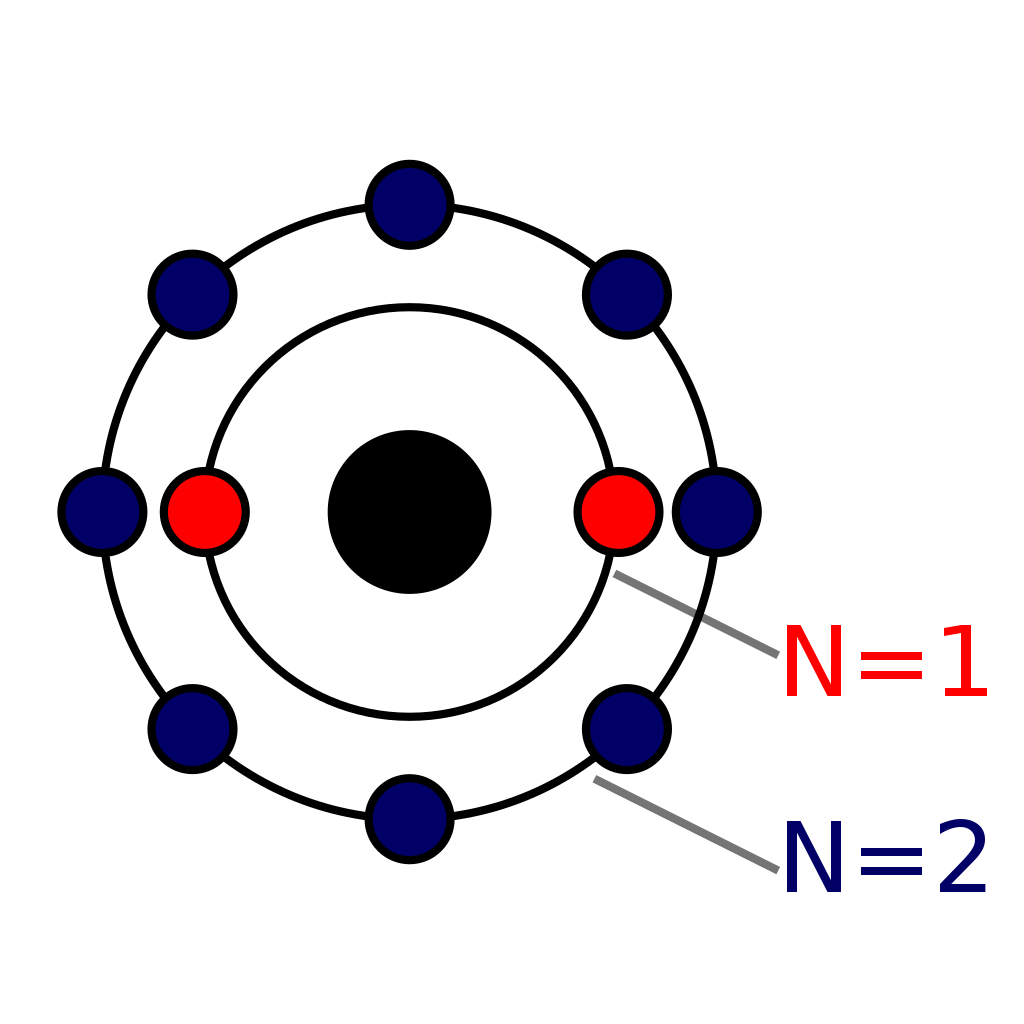
Neon Bohr Diagram
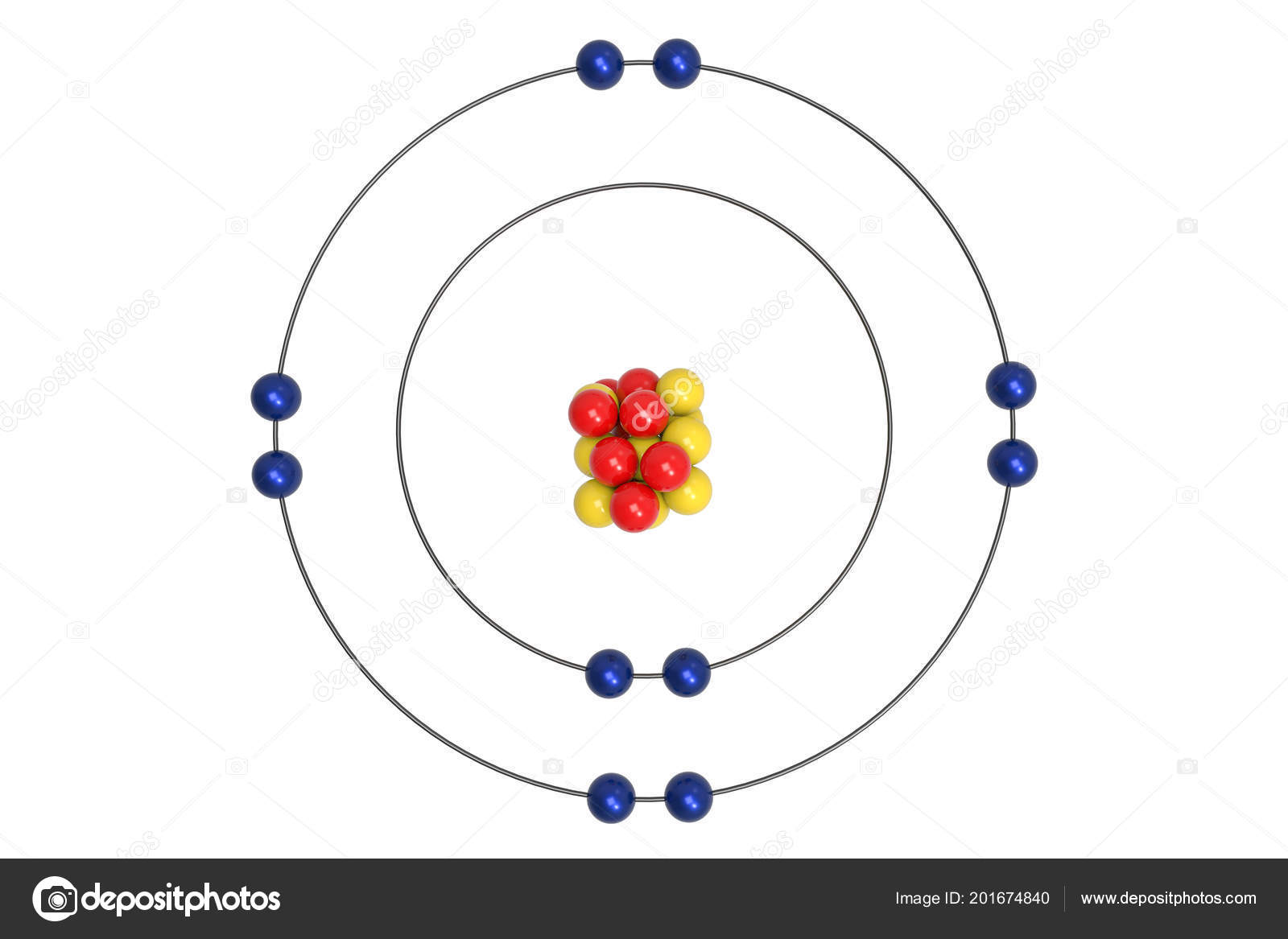
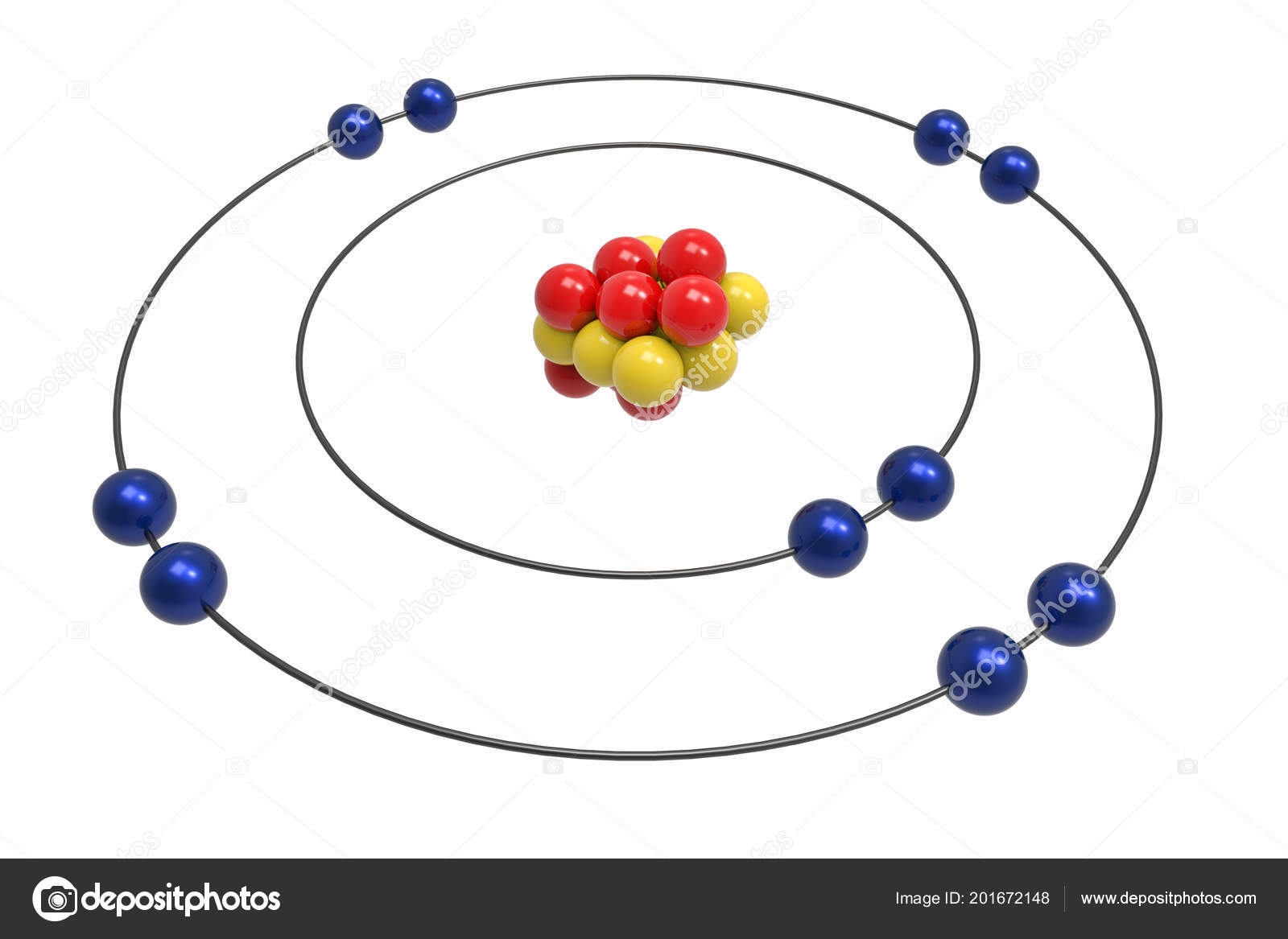
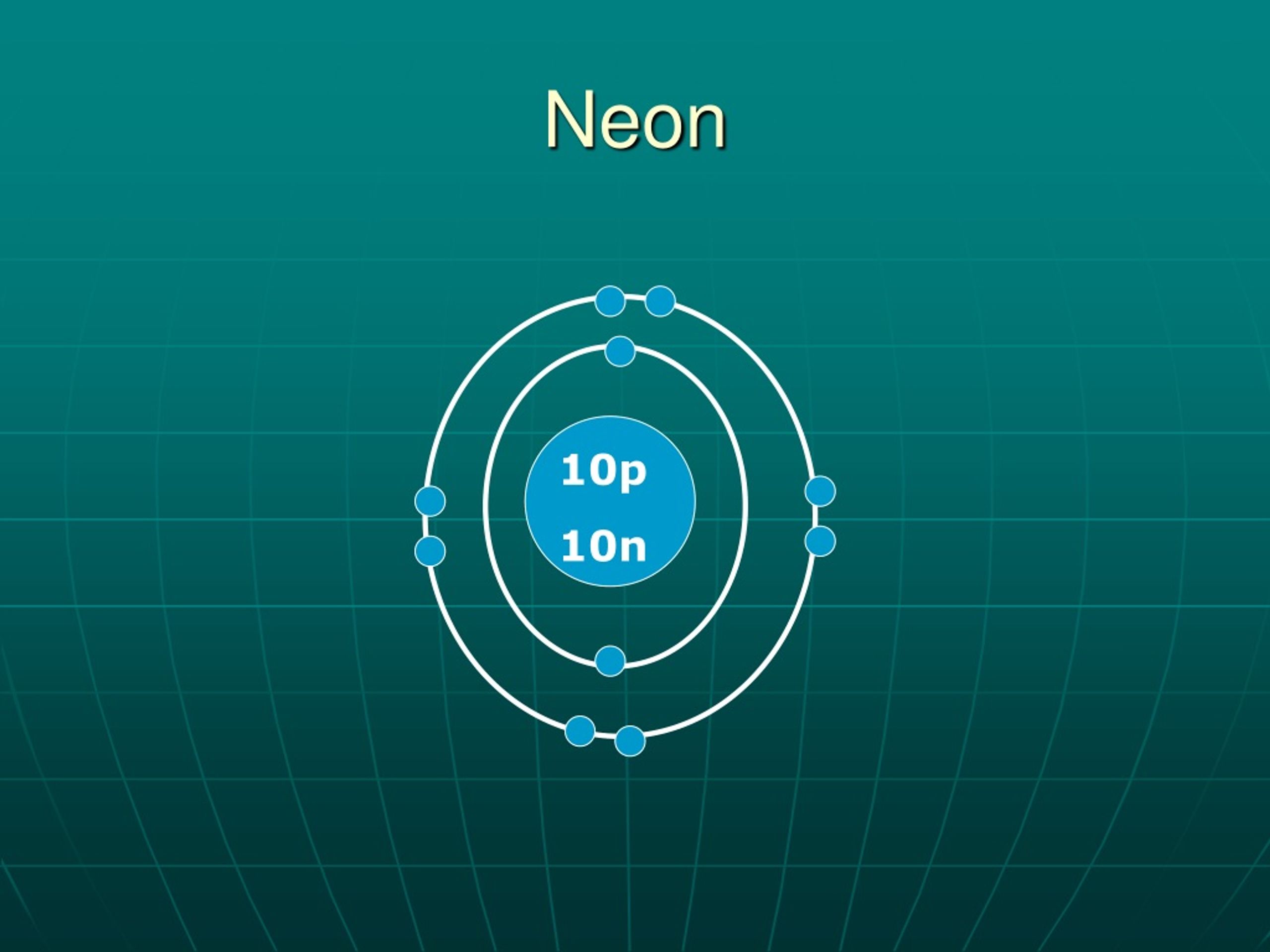
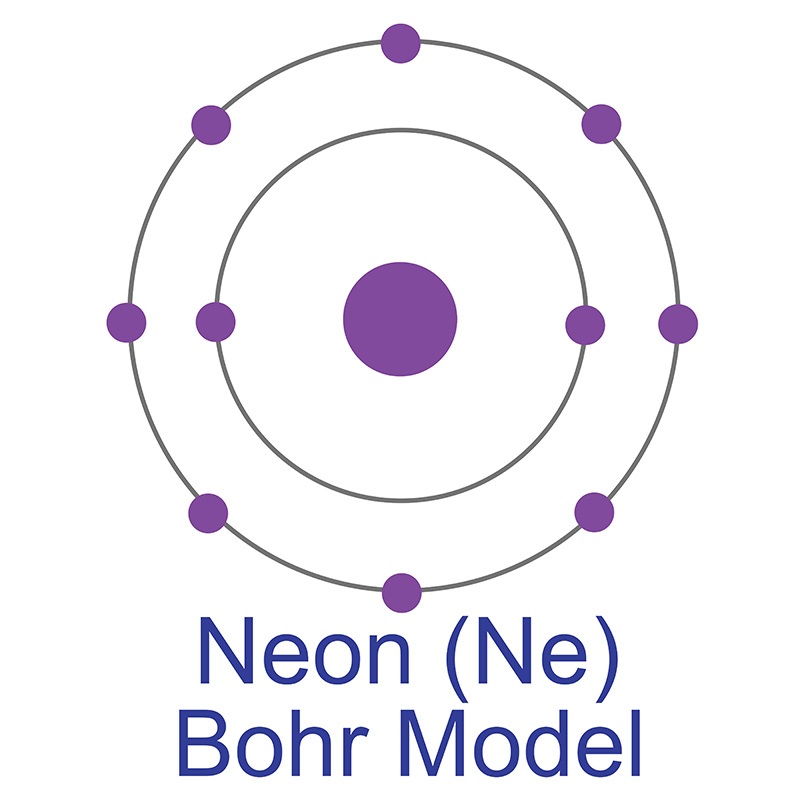
The Bohr Model of Neon (Ne) has a nucleus that contains 10 neutrons and 10 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Neon contains 8 electrons that also called valence electrons. Page Contents show How to draw Bohr Model of Neon (Ne)?

Neon Bohr Model — Diagram, Steps To Draw Techiescientist
Rutherford-Bohr diagram Lewis notation If an element has more than four valence electrons, they are doubled to form pairs of dots around the element. For example, neon is represented by eight valence electrons. Rutherford-Bohr diagram Lewis notation SAMPLE QUESTIONS: 1) Draw a Lewis structure for each of the following elements: Potassium
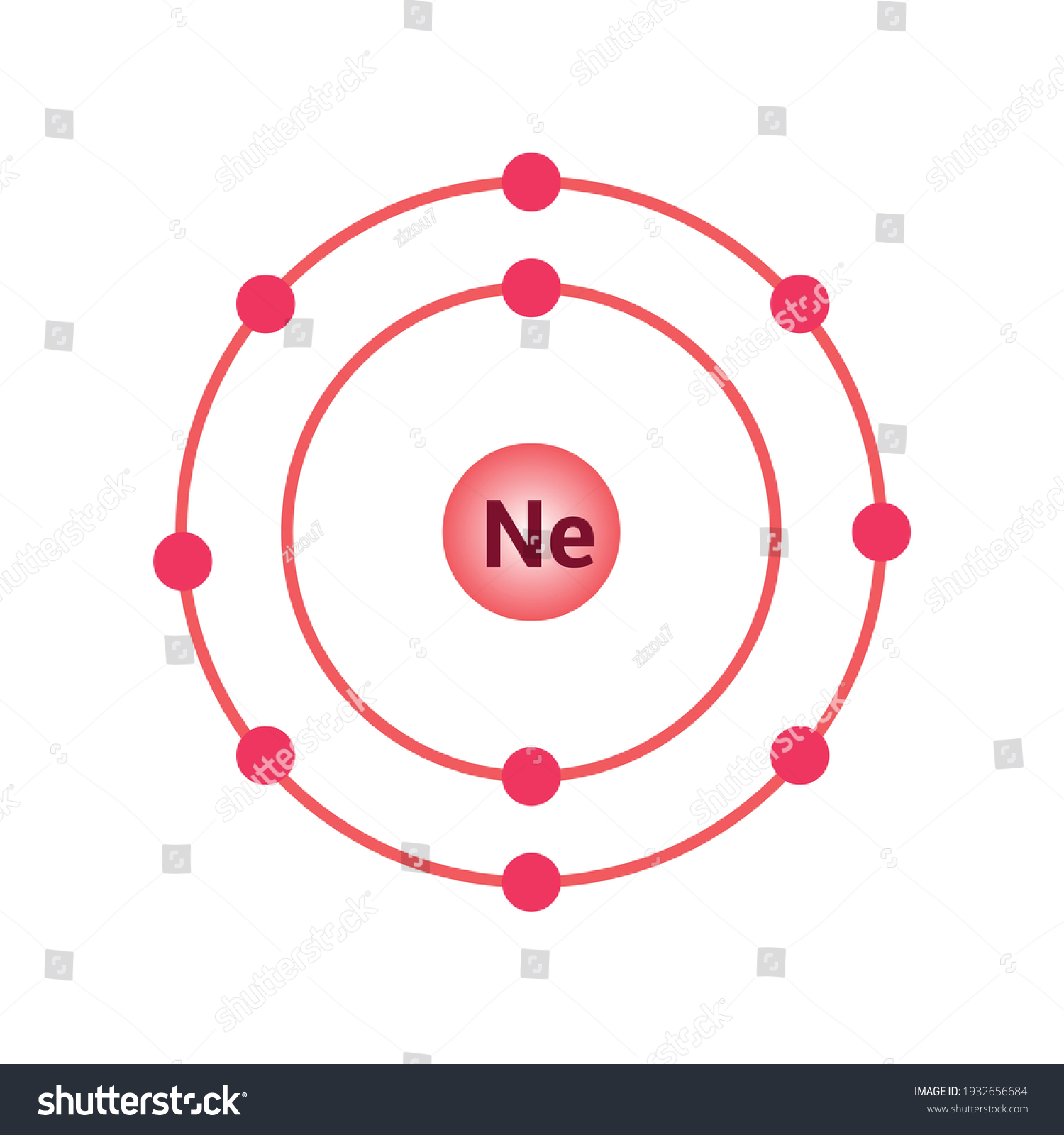
modelo bohr del átomo de neón. vector de stock (libre de regalías
Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. This is because protons and neutrons.

Schematic Representation Of Atoms Model Making School Project Bohr
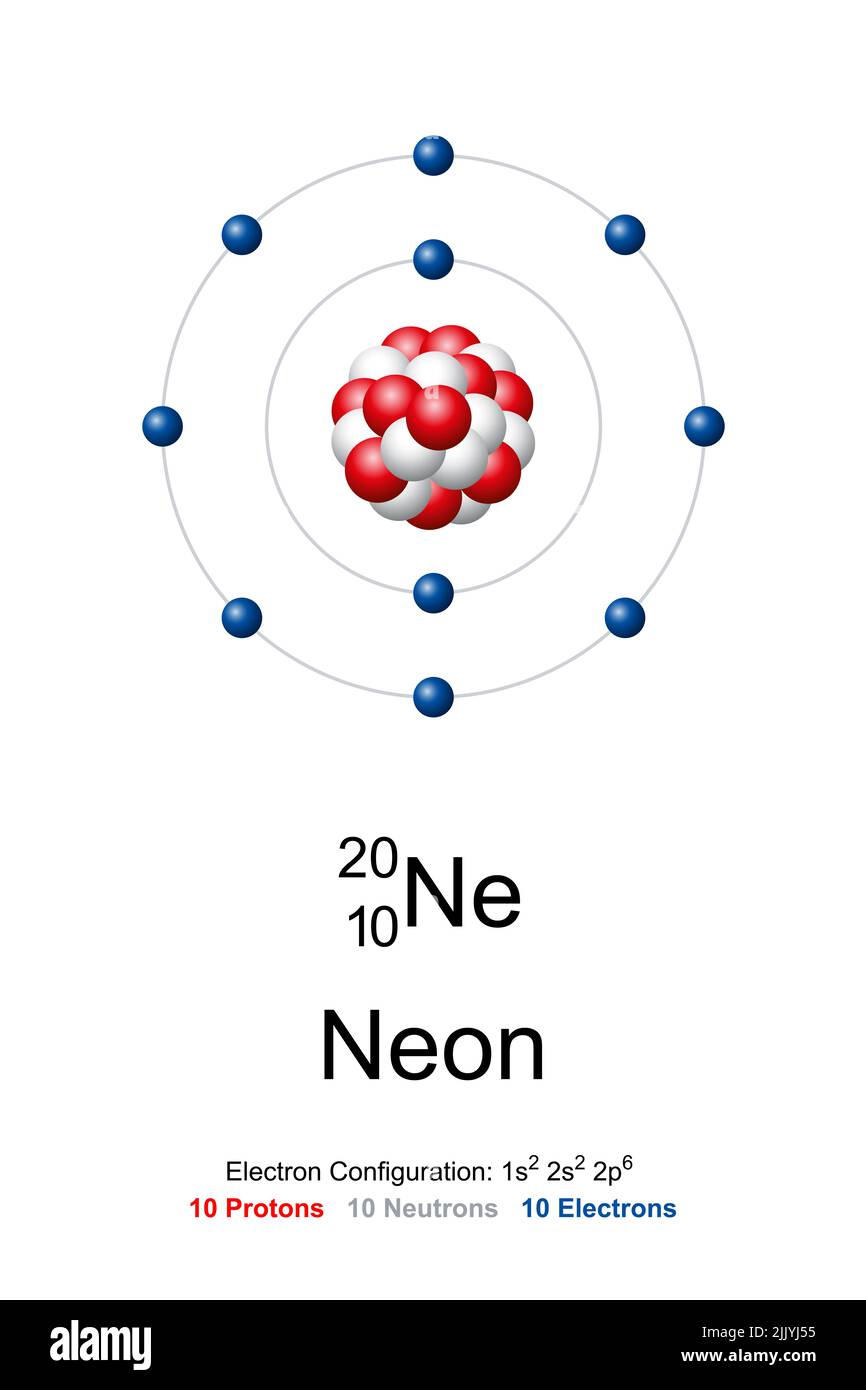
The Rutherford-Bohr atomic model is a representation of the atom as a very small. Neon Atomic # 10 Atomic mass # 20 10 electrons distributed like this: - 2 on the first level - 8 on the second level RUTHERFORD- BOHR DIAGRAM . VALENCE ELECTRONS

The Rutherford atomic model of a neon atom, showing the atom as similar
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). You will get the detailed information about the periodic table which will convert a newbie into pro. 3). You will also get the HD images of the Periodic table (for FREE).
Bohr Model Labeled
Figure 2.5. 1: Bohr diagrams: Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially-filled valence shells and gain or lose electrons to.
Bohr Model Neon
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.
Neon Atom Bohr Model Proton Neutron Electron Illustration Stock Photo
The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to.
Picture bohr model for neon Bohr Model Neon Atom Proton Neutron
Atomic Structure (Bohr Model) for the Neon (Ne) Atom Wayne Breslyn 718K subscribers Join Subscribe Subscribed 35 Share 5.7K views 1 year ago In this video we'll look at the atomic structure and.
PPT BohrRutherford Diagrams PowerPoint Presentation, free download
A detailed look at the Bohr -Rutherford Diagram for Phosphorus is provided below. The box on the left provides the Chemical Symbol (P), the Atomic. Neon Chlorine 40 Ca 20 11 B 5 20 Ne 10 35 Cl 17. Title: Unit 2 Lesson 5 Bohr-Rutherford Diagrams 2.PDF Author: Rm-227 Created Date:
Lewis Dot Diagram For Neon
In atomic physics, the Bohr model or Rutherford-Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons.
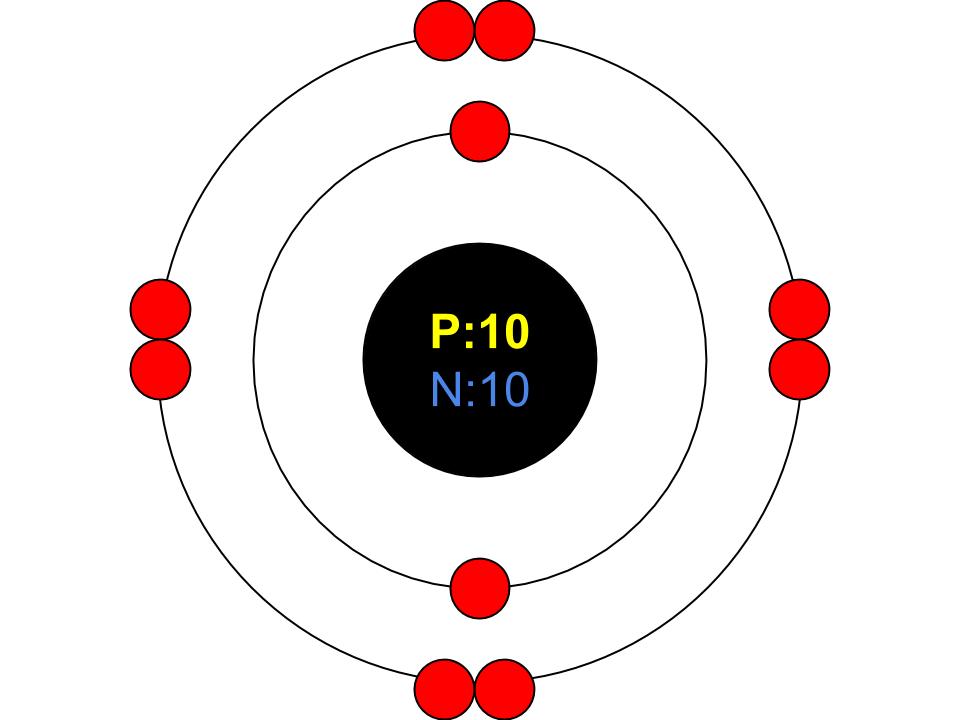
Bohr Model Neon
Bohr Model of Atoms. Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. This picture was called the planetary model, since it pictured the atom as a miniature "solar system" with the electrons.

Bohrs atom model Bohrs atom model The neon
These are represented by the symbol p +. • Neutrons: These are the charge-neutral particles located inside the nucleus. These are represented using the symbol n°. • Electrons: These are the negatively charged atomic particles that revolve around the nucleus. The electrons follow a definite path while revolving around the nucleus.